Page 114 - A4_Adelphi_bed_extras_brochure_20182
P. 114
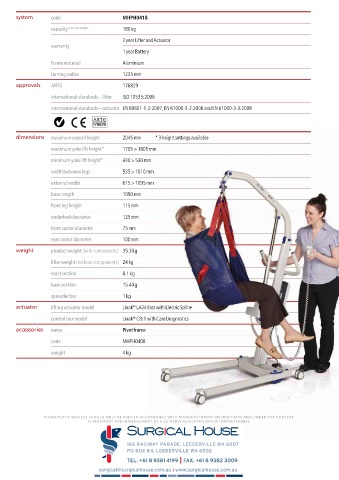
system code MHPH0418
capacity max user weight 180 kg
2 year Lifter and Actuator
warranty
1 year Battery
frame material Aluminium
turning radius 1235 mm
approvals ARTG 176829
international standards – lifter ISO 10535:2006
international standards – actuator EN 60601-1-2:2007, EN 61000-3-2:2006 and EN 61000-3-3:2008
176829
dimensions maximum overall height 2045 mm * 3 height settings available
maximum yoke lift height* 1705 > 1805 mm
minimum yoke lift height* 430 > 530 mm
width between legs 535 > 1010 mm
external width 615 > 1095 mm
base length 1090 mm
front leg height 115 mm
underbed clearance 125 mm
front castor diameter 75 mm
rear castor diameter 100 mm
weight product weight (with components) 35.3 kg
lifter weight (without components) 24 kg
mast section 8.1 kg
base section 15.4 kg
spreader bar 1 kg
actuator lifting actuator model Linak LA34 Fast with Electric Spline
®
control box model Linak CBJ1 with Care Diagnostics
®
accessories name Pivot frame
code MHPH0408
weight 4 kg
TherapeuTic devices should only be used in accordance wiTh manufacTurers insTrucTions and under The consenT,
supervision and managemenT of a suiTably qualified healTh professional.